- TEL:
- +81-3-5462-4831
- FAX:
- +81-3-5462-4835
※9:00-17:40 Mon.-Fri. (JST)


Hiroshi Arakawa, Daichi Higuchi, Etsushi Takahashi, Kohei Matsushita, Shiho Nedachi, Hanwei Peng, Moeno Kadoguchi,
Kaoru Morimura, Ayano Araki, Masayuki Kondo, Naoki Ishiguro, Yoichi Jimbo and Ikumi Tamai
J. Pharm. Sci. 113, 3255 - 3264 (2024). https://doi.org/10.1016/j.xphs.2024.08.009
Copyright © Authors 2024
This article is licensed under a Creative Commons Attribution 4.0 International License (CC BY).
Drug-induced kidney injury (DIKI) is a major cause of failure in drug discovery and development. Animal experiments are being conducted to evaluate DIKI, but due to their low sensitivity, the development of human in vitro systems with a high predictive ability is desirable for drug discovery and development.
Renal proximal tubule epithelial cells (RPTEC) are useful for evaluating RPTEC injury in vitro, but conventional two-dimensional culture models show low expression of drug transporters and are not suitable for the evaluation of DIKI mediated by a wide range of drug transporters.
Research AchievementsThe group constructed 3D-RPTEC spheroids with higher OAT1 mRNA expression, attaining levels comparable with those in human kidney cortices. In this study, they investigated the utility of 3D-RPTECs for evaluating RPTEC injury by monitoring intracellular ATP levels as a cytotoxic indicator. The predictive performance of 3D-RPTECs was higher than that of two-dimensional cultured RPTECs and the kidney cell line HK-2. In conclusion, 3D-RPTECs are useful for in vitro evaluation of RPTEC injury by measuring intracellular ATP levels. Use of PrimeSurface™ in this study
|
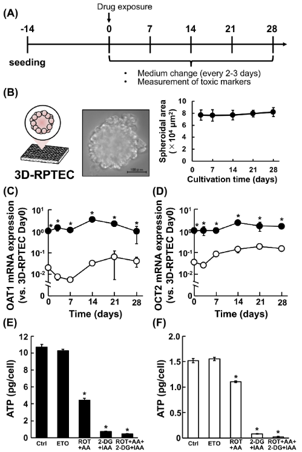
Characterization of 3D-RPTEC compared with 2D culture |
| Cat # | Product name | Well | Color | Bottom design | Well Vol | Package |
|---|---|---|---|---|---|---|
| MS-9096VZ | PrimeSurface™ 96V | 96 | Transparent | V bottom | 300 μL | Individual packaging 20 plates per case |
Remark