- TEL:
- +81-3-5462-4831
- FAX:
- +81-3-5462-4835
※9:00-17:40 Mon.-Fri. (JST)

|
PROTEOSAVE™ is a product that blocks nonspecific adsorption of protein, peptide to the inside of a plastic container by the coating of ultra hydrophilic polymer that S-BIO has newly and originally developed. |
The unique surface hydroxide group is coating and decreasing the loss of protein sample by preventing non-specific adsorption of protein and peptide.
PROTEOSAVE™ is stable against general used organic solvents, detergents and heating below 100°C.
Adjustment/Preservation
| Cat. No | Product | Material | Note | Qty/Pk | Qty/Cs |
|---|---|---|---|---|---|
| MS-4205M | PROTEOSAVE™ Microtube 0.5mL | Polypropylene | Non-sterilized | 100 | 500 |
| MS-4255M | PROTEOSAVE™ Microtube 0.5mL R (Sterilized) | Polypropylene | Radiation sterilized | 100 | 500 |
| MS-4215M | PROTEOSAVE™ Microtube 1.5mL | Polypropylene | Non-sterilized | 100 | 500 |
| MS-4265M | PROTEOSAVE™ Microtube 1.5mL R (Sterized) | Polypropylene | Radiation sterilized | 100 | 500 |
| MS-4220M | PROTEOSAVE™ Microtube 2.0mL | Polypropylene | Non-sterilized | 100 | 500 |
| MS-4270M | PROTEOSAVE™ Microtube 2.0mL R (Sterized) | Polypropylene | Radiation sterilized | 100 | 500 |
| MS-4201X | PROTEOSAVE™ Slimtube 0.5mL | Polypropylene | Non-sterilized | 50 | 500 |
| MS-4202X | PROTEOSAVE™ Slimtube 1.5mL | Polypropylene | Non-sterilized | 50 | 500 |
Remark
| Cat. No | Product | Material | Note | Qty/Pk | Qty/Cs |
|---|---|---|---|---|---|
| MS-52150* | PROTEOSAVE™ Conicaltube 15mL | Body: PET Cap: Polyethylene | Non-sterilized | 5 | 100 |
| MS-52550 | PROTEOSAVE™ Conicaltube 50mL R (Sterilized) | Body: Polypropylene Cap: Polyethylene | Radiation sterilized | 5 | 100 |
| MS-8296F | PROTEOSAVE™ 96-wells, Flat Plate | Polystyrene | No Lid, Non-sterilized | 5 | 50 |
| MS-8296V | PROTEOSAVE™ 96-wells, V Plate | Polypropylene | No Lid, Non-sterilized | 5 | 20 |
| MS-82962R | PROTEOSAVE™ 96-deep wells, V Plate, 2mL | Polypropylene | Radiation sterilized | 3 | 15 |
| MS-8296K | PROTEOSAVE™ 96-wells, Black, Flat Plate | Polystyrene | No Lid, Non-sterilized | 5 | 50 |
| MS-3296U | PROTEOSAVE™ 96-wells, U Plate | Polystyrene | No Lid, Non-sterilized | 5 | 50 |
Remark
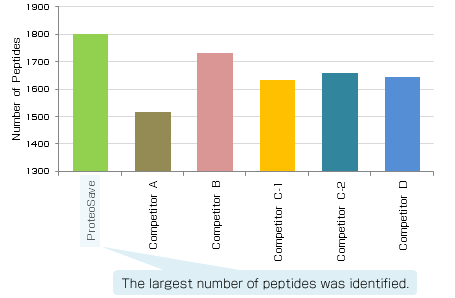
|
Experimental conditions
|
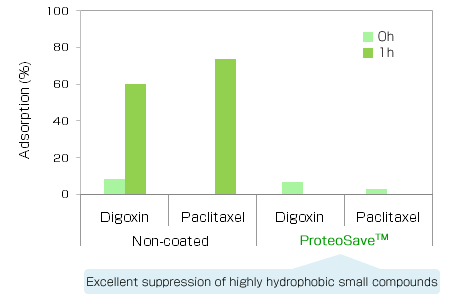
|
Experimental conditions
Adsorption (%) = 100 - Concentration in the 0 and 1h sample / Concentration in the initial sample x 100
|
| 1. | YOSHIDA, Mitsutaka, et al. Preferential capture of EpCAM-expressing extracellular vesicles on solid surfaces coated with an aptamer-conjugated zwitterionic polymer. Biotechnology and bioengineering, 2018, 115.3: 536-544. |
|---|---|
| 2. | ARISAKA, Yoshinori, et al. A heparin-modified thermoresponsive surface with heparin-binding epidermal growth factor-like growth factor for maintaining hepatic functions in vitro and harvesting hepatocyte sheets. Regenerative Therapy, 2016, 3: 97-106. |
| 3. | PANDEY, Kiran; NAHAR, Ashrafun; KADOKAWA, Hiroya. Method for isolating pure bovine gonadotrophs from anterior pituitary using magnetic nanoparticles and anti-gonadotropin-releasing hormone receptor antibody. Journal of Veterinary Medical Science, 2016, 78.11: 1699-1702. |
| 4. | HAMAMURA, Kensuke, et al. ANNALS EXPRESS: Simple quantitation for potential serum disease biomarker peptides, primarily identified by a peptidomics approach in the serum with hypertensive disorders of pregnancy. Annals of Clinical Biochemistry: An international journal of biochemistry and laboratory medicine, 2015, 0004563215583697. |
| 5. | IZAKI, Shunsuke, et al. Feasibility of Antibody-Poly (Glutamic Acid) Complexes: Preparation of High-Concentration Antibody Formulations and Their Pharmaceutical Properties. Journal of pharmaceutical sciences, 2015, 104.6: 1929-1937. |
| 6. | OGISO, Hideo; TANIGUCHI, Makoto; OKAZAKI, Toshiro. Analysis of lipid-composition changes in plasma membrane microdomains. Journal of lipid research, 2015, 56.8: 1594-1605. |
| 7. | ICHIKAWA, Shunsuke, et al. Cellulosomal carbohydrate-binding module from Clostridium josui binds to crystalline and non-crystalline cellulose, and soluble polysaccharides. FEBS letters, 2014. |
| 8. | KADOKAWA, Hiroya, et al. Gonadotropin-releasing hormone (GnRH) receptors of cattle aggregate on the surface of gonadotrophs and are increased by elevated GnRH concentrations. Animal reproduction science, 2014, 150.3: 84-95. |
| 9. | UCHIDA, Yasuo, et al. A study protocol for quantitative targeted absolute proteomics (QTAP) by LC-MS/MS: application for inter-strain differences in protein expression levels of transporters, receptors, claudin-5, and marker proteins at the blood-brain barrier in ddY, FVB, and C57BL/6J mice. Fluids and Barriers of the CNS, 2013, 10.1: 21. |
| 10. | NAGAI, Yutaka; TAKAO, Masashi. Monoclonal antibody to human epithelial cell adhesion molecule and method for detecting circulating tumor cells using the same. U.S. Patent Application 14/085,205, 2013. |
| 11. | TAKAO, Masashi; NAGAI, Yutaka; TORII, Tokiji. Cysteine-Poor Region-Specific EpCAM Monoclonal Antibody Recognizing Native Tumor Cells with High Sensitivity. Monoclonal antibodies in immunodiagnosis and immunotherapy, 2013, 32.2: 73-80. |
| 12. | YASUNO, K., et al. Development of Podocyte Injuries in Osborne-Mendel Rats is Accompanied by Reduced Expression of Podocyte Proteins. Journal of comparative pathology, 2013, 149.2: 280-290. |
| 13. | TSUCHIYA, Hikaru; TANAKA, Keiji; SAEKI, Yasushi. The parallel reaction monitoring method contributes to a highly sensitive polyubiquitin chain quantification. Biochemical and biophysical research communications, 2013, 436.2: 223-229. |
| 14. | KUROKAWA, Kenji, et al. Novel bacterial lipoprotein structures conserved in low-GC content Gram-positive bacteria are recognized by Toll-like receptor 2. Journal of Biological Chemistry, 2012, 287.16: 13170-13181. |
| 15. | UMEMURA, Hiroshi, et al. Identification of a high molecular weight kininogen fragment as a marker for early gastric cancer by serum proteome analysis. Journal of gastroenterology, 2011, 46.5: 577-585. |
| 16. | KAWAKAMI, Hirotaka, et al. Dynamics of absolute amount of nephrin in a single podocyte in puromycin aminonucleoside nephrosis rats calculated by quantitative glomerular proteomics approach with selected reaction monitoring mode. Nephrology Dialysis Transplantation, 2011, gfr492. |
| 17. | TAKAO, Masashi; TAKEDA, Kazuo. Enumeration, characterization, and collection of intact circulating tumor cells by cross contamination-free flow cytometry. Cytometry Part A, 2011, 79.2: 107-117. |
| 18. | FUKUMOTO, Hiroaki, et al. High-molecular-weight β-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. The FASEB Journal, 2010, 24.8: 2716-2726. |
| 19. | ICHIKAWA, S.; KARITA, S. Characterization of Family 3 Carbohydrate-binding Module from Clostridium josui. In: Proceedings of the Second International Workshop on Regional Innovation Studies: (IWRIS2010). Graduate School of Regional Innovation Studies, Mie University, 2010. p. 5-8. |
| 20. | HONJO, Megumi, et al. Autotaxin-lysophosphatidic acid pathway in intraocular pressure regulation and glaucoma subtypes. Investigative ophthalmology & visual science, 2018, 59.2: 693-701. |
|---|
| 21. | MIZUI, Toshiyuki, et al. Cerebrospinal fluid BDNF pro-peptide levels in major depressive disorder and schizophrenia. Journal of psychiatric research, 2019, 113: 190-198. |
|---|---|
| 22. | OHARA, Yuki; YOSHIMOTO, Shogo; HORI, Katsutoshi. Control of AtaA-mediated bacterial immobilization by casein hydrolysates. Journal of bioscience and bioengineering, 2019, 128.5: 544-550. |
| 23. | ISHII, Takashi, et al. Increased cerebrospinal fluid complement C5 levels in major depressive disorder and schizophrenia. Biochemical and biophysical research communications, 2018, 497.2: 683-688. |
| 24. | MATSUMURA, Takayuki, et al. Venom and Antivenom of the Redback Spider (Latrodectus hasseltii) in Japan. Part I. Venom Extraction, Preparation, and Laboratory Testing. Japanese journal of infectious diseases, 2018, 71.2: 116-121. |
| 25. | IGARASHI, Nozomi, et al. Increased aqueous autotaxin and lysophosphatidic acid levels are potential prognostic factors after trabeculectomy in different types of glaucoma. Scientific reports, 2018, 8.1: 1-13. |
| 26. | HIDESE, Shinsuke, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2017, 76: 12-18. |
| 27. | YOSHIMOTO, Shogo, et al. An Acinetobacter trimeric autotransporter adhesin reaped from cells exhibits its nonspecific stickiness via a highly stable 3D structure. Scientific Reports, 2016, 6. |
| 28. | HATTORI, Kotaro, et al. Increased cerebrospinal fluid fibrinogen in major depressive disorder. Scientific reports, 2015, 5: 11412. |
| 29. | WATANABE, H., et al. Controlled release of a protein using a ceramic carrier and zinc ions as a novel approach to the treatment of osteoporosis. In: Key Engineering Materials. 2015. p. 332-337. |
| 30. | IHARA, Yuta; OHTA, Hiroyuki; MASUDA, Shinji. A highly sensitive quantification method for the accumulation of alarmone ppGpp in Arabidopsis thaliana using UPLC-ESI-qMS/MS. Journal of plant research, 2015, 128.3: 511-518. |
| 31. | KIM, Jong-Myong, et al. Highly Reproducible ChIP-on-Chip Analysis to Identify Genome-Wide Protein Binding and Chromatin Status in Arabidopsis thaliana. In: Arabidopsis Protocols. Humana Press, 2014. p. 405-426. |
| 32. | FURUGORI, Taketoshi; MORISHIMA, Yoshiyuki. PHARMACEUTICAL COMPOSITION FOR PROMOTION OF FIBRINOLYSIS. U.S. Patent Application No 15/538,676, 2017. |
|---|---|
| 33. | FUKAZAWA, Tominaga; YAMAZAKI, Yuri; MIYAMOTO, Yohei. Reduction of non-specific adsorption of drugs to plastic containers used in bioassays or analyses. Journal of pharmacological and toxicological methods, 2010, 61.3: 329-333. |
| 34. | OBAYASHI, Yumiko; WEI BONG, Chui; SUZUKI, Satoru. Methodological Considerations and Comparisons of Measurement Results for Extracellular Proteolytic Enzyme Activities in Seawater. Frontiers in microbiology, 2017, 8: 1952. |
|---|---|
| 35. | ANDOU, Takashi, et al. RNA detection using peptide-inserted Renilla luciferase. Analytical and bioanalytical chemistry, 2009, 393.2: 661-668. |
| 36. | SAMESHIMA-YAMASHITA, Yuka, et al. Construction of a Pseudozyma antarctica strain without foreign DNA sequences (self-cloning strain) for high yield production of a biodegradable plastic-degrading enzyme. Bioscience, biotechnology, and biochemistry, 2019, 83.8: 1547-1556. |
|---|---|
| 37. | KINOSHITA, Shigeru, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. New England Journal of Medicine, 2018, 378.11: 995-1003. |
| 38. | KOBAYASHI, Jun, et al. Effect of temperature changes on serum protein adsorption on thermoresponsive cell-culture surfaces monitored by a quartz crystal microbalance with dissipation. International journal of molecular sciences, 2018, 19.5: 1516. |
| 39. | KUBOTA, Hiroyuki, et al. Reduction in IgE reactivity of Pacific mackerel parvalbumin by heat treatment. Food chemistry, 2016, 206: 78-84. |
| 40. | KASUGA, Kie. Comprehensive analysis of MHC ligands in clinical material by immunoaffinity-mass spectrometry. In: The Low Molecular Weight Proteome. Springer New York, 2013. p. 203-218. |
| 41. | YAMASHITA, Kazuyuki; SHIROKI, Masahiro. Medical or biochemical resin composition and resin molded product. U.S. Patent Application 13/469,768, 2012. |
| 42. | GOTOH, Akiko, et al. Evaluation of adsorption of urine cystatin C to the polymer materials on the microplate by an antigen capture enzyme-linked immunosorbent assay. Clinica Chimica Acta, 2008, 397.1: 13-17. |