The Group‘s Basic Policy and System for Quality Assurance
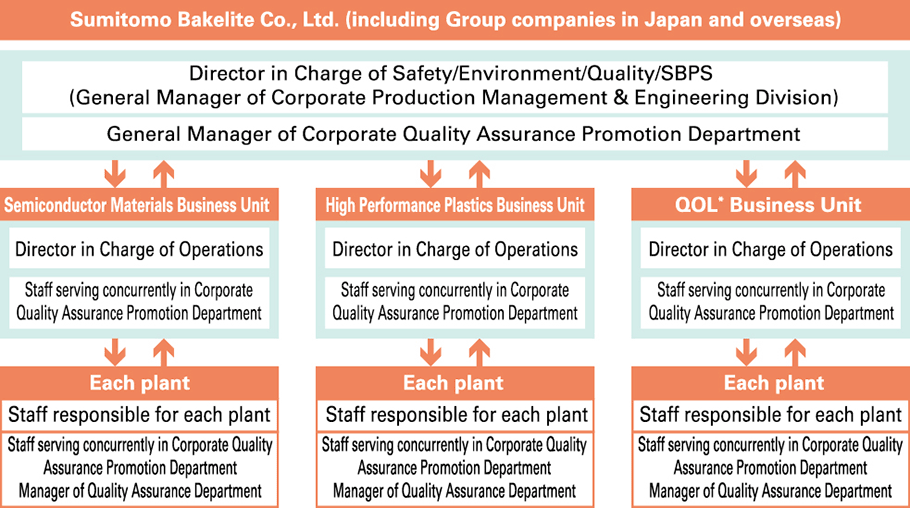
Our Group has established quality management systems (QMS) based on ISO 9001 and is continuing to acquire relevant certifications (a total of 41 sites have been certified as of May 1, 2023). Recognizing the provision of products and services that customers can always feel satisfaction and peace of mind in using is an important social mission for our Group, and all relevant departments collaborate on all processes—from product planning, research, design & development, preparation for production, production, sales & service, to quality assurance—with an awareness of the importance of ensuring the safety of products to create and appropriately implement and manage frameworks within which to enhance and maintain product safety and quality. In order to ensure that all employees of our Group systematically implement product safety and quality assurance initiatives in accordance with QMS, we have formulated a Quality Control Policy and provide education to quality control manager candidates as part of our Quality Management Representative Training Course.
●Quality Management System
- See the website glossary page.
Fiscal 2023 quality management policy
Basic policy
By making essential improvements from creating a good flow of quality formation with customer first and quality first in mind and contribute to increasing profits, while at the same time promoting SDGs and contributing to society.
One Sumibe / Zero Defect / Proactive
Measures: SDGs 12: Ensure sustainable consumption and production patterns
- 1. Working toward Ensuring Quality that Provides Safety and Security of Mind (QA Department’s Role and Responsibility)
- 2. Quality Improvement Activities of Existing Businesses (Complaints Handling Towards at Improving Customer Satisfaction, Reduction of F Costs)
- 3. Risk Reduction of New Products and New Businesses
- 4. Improvement of the Entire Total Manufacturing (Monozukuri) Process through Daily Inspection and ‘Monozukuri’ Audit
- 5. Training Quality Management Representative who takes on the Next Generation
●QMS Certification Received
| Certification standard | Business/products |
|---|---|
| ISO9001 | Quality-of-life products (packaging films for food and pharmaceutical products, bio-based products, construction materials, waterproofing products, etc.) |
| High Performance Plastics (included molded articles) |
|
| Semiconductor Materials | |
| IATF16949 | High Performance Plastics (included molded articles) |
| Semiconductor Materials | |
| Thermoplastic sheets | |
| ISO13485 | Medical devices, in-vitro diagnostics |
| ISO15378 | Packaging Materials for Pharmaceuticals |
| FSSC22000 | Packaging films for food |
| AS 9100 or JIS Q 9100 |
Aircraft components |
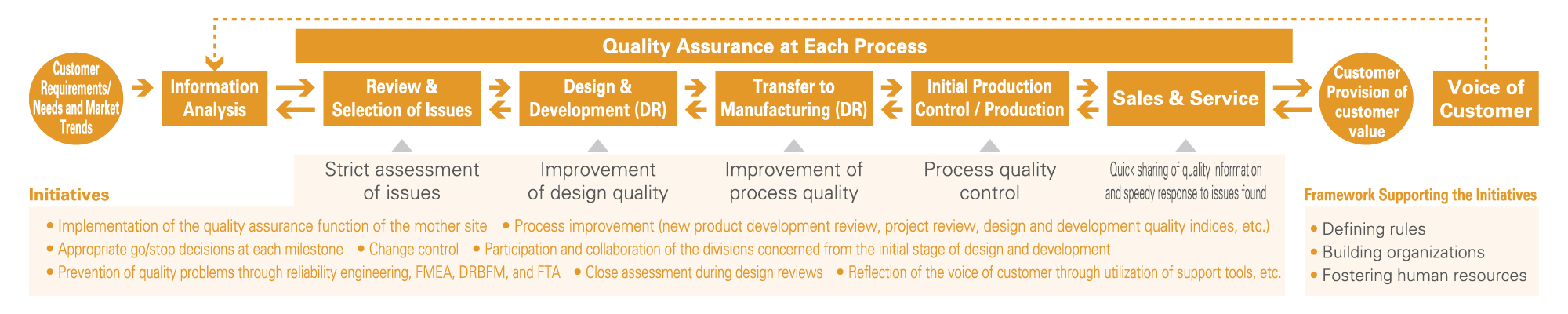
●Future State Vision of Appropriate New-Product Development and Commercialization Processes of the Group
Quality Improvement Activities for Existing Business
We are working to enhance the quality of our existing products through such efforts as ensuring rapid response to complaints, taking measures to prevent recurrences of problems, and rigorous 4M change control. Cross-functional responses are made to rapidly solve not only serious but also minor complaints. In order to prevent new occurrences and recurrence of issues leading to complaints and process abnormality, we use methods like “Why-Why Analysis” and “Further Investigation” to identify what happened, causes, and countermeasures, among other factors, concerning these issues. From fiscal 2022, we have been promoting integrated management of complaints for new businesses too and collaborating on reduction activities, and will continue to do so in fiscal 2023.
Reducing Risk Relating to New Business
There is a need to improve (optimize) the output quality (degree of perfection) of product designs and process designs when developing new products and to shorten (minimize) the time required for the development process by minimizing rework. In order for our Group to meet these needs, we continually make efforts toward improvement and refinement our QMS from the standpoint of functional assurance.
❶ Shortening New-Product Development Periods and Improving Work Quality
In new product development, initial plans are often delayed because of the tendency for a variety of problems requiring reworking to arise. To prevent this, we implement the Plan-Do-Check-Action (PDCA) cycle to increase the degree of perfection of design quality and shorten the development period through collaboration of all the divisions concerned from the initial phases. Furthermore, we implement the following to ensure that the problems do not recur in subsequent development work.
| (1) | Feedback Review Analysis to identify problems through reviews of development processes over time. |
|---|---|
| (2) | Why-Why Analysis and Further Investigation to identify root causes of the occurrence and outflowing of problems in terms of technology and management. Why-Why Analysis and Further Investigation are also used to determine why problems were not prevented in terms of organizations, allocation of functions, systems, frameworks, and culture and to identify measures for preventing recurrence and new occurrences. |
❷ Proactive Use of Various Quality Control Techniques
In addition to design review (DR) during each stage of product design and process design, we conduct Failure Modes and Effects Analysis (FMEA) to predict potential failures or abnormalities by analyzing health and safety risks regarding people including customers related to our products, along with Design Review Based on Failure Mode (DRBFM) that focuses on changes to the design and changes to conditions and the environment. In turn, we implement risk reduction measures in all processes of DR, FMEA, and DRBFM as well as during technical verification at the time of using new raw materials. In addition, we use Fault Tree Analysis (FTA) that rationally analyzes accidents and defects in a hierarchical manner to discover root causes and fundamental solutions for preventing recurrence.
In foscal 2022, our approach to functional assurance (functional block diagram with elementary blocks) has been added to quality training for young employees as activities to reduce missed extracts (exceptions) during failure mode. We will continue these educational efforts in fiscal 2023.
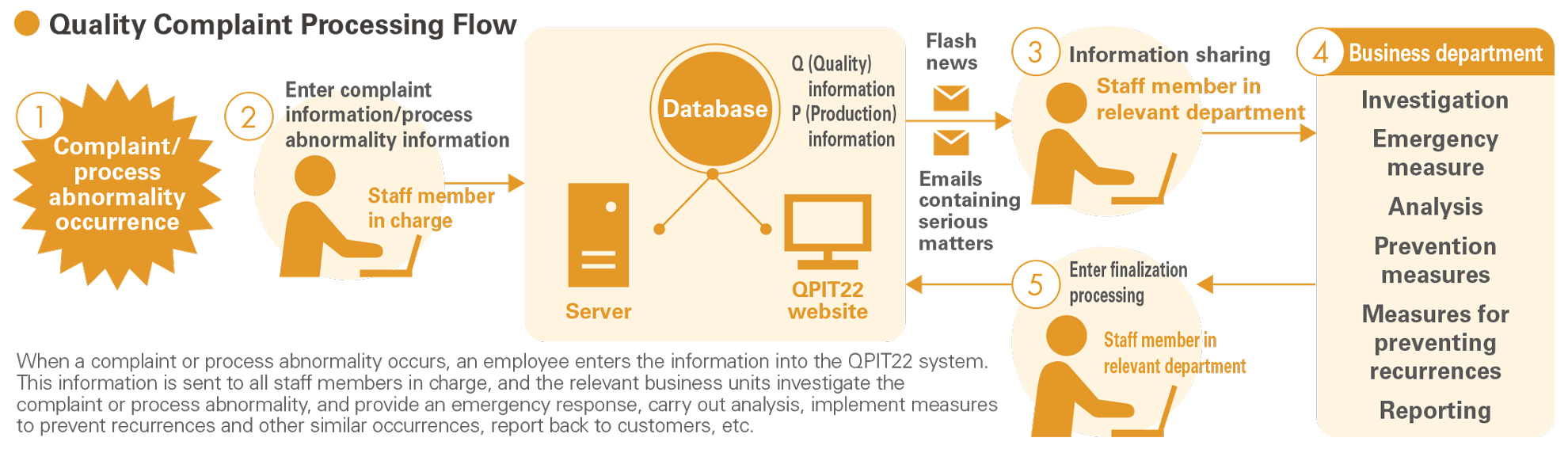
The “QPIT” System for Processing Quality-Related Complaints
We manage complaints relating to quality using the “QPIT” system. QPIT (Quality & Production Information Tools) is a system that allows the central management of quality- and production-related information, and it has been built into the Groupwide intranet. The system was introduced and utilized in order to accelerate the communication of quality- and production-related information within the Group, facilitate the sharing of information quickly with management, and encourage the integration and effective utilization of information levels. We can provide feedback of complaint statistics based on QPIT information to business units in order to confirm the effectiveness of claim recurrence prevention and to shorten the time required to respond to claims, ensuring quick and accurate handling of complaints in an efficient manner.
We are utilizing various kinds of information stored in the QPIT, like details of complaints and customer requests, to enhance CS. Similar complaints and customer requests, and cases from other department businesses can be used as reference, and by analyzing the content and trends of such information and implementing measures to address the issues while building a framework to improve these and sharing these internally, a wide range of divisions can work to achieve the aim of improving CS.
We rolled out and began using the new QPIT22 system in fiscal 2022, which is used for statistical analysis of the complaints database that is compiled for data record purposes. We have also begun creating a centralized data dashboard to share quality information across the group.
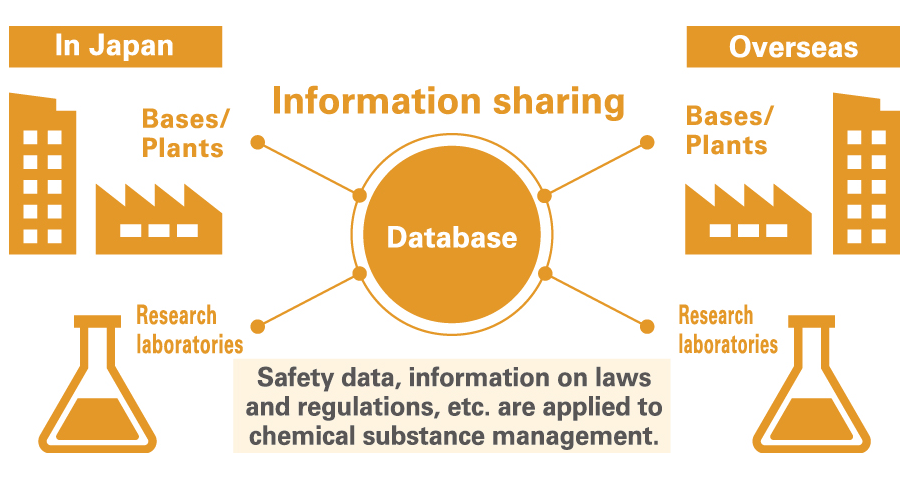
Chemical Substance Management System
In the interest of ensuring safety, we confirm that all of the chemical substances constituting the raw materials and products handled by the Group conform with the laws and regulations of each country. We are also making progress with the creation of a Chemical Substance Management System to centrally manage these related chemicals. Introducing this system allows us to speed up chemical substance-related investigations (inventory in each country, the safety of products and raw materials, regulatory information, etc.) and to provide accurate information. We are now rolling out the system laterally to plants that manufacture molded articles*1 and expanding application of volume tracking management to products for Taiwan and South Korea, in addition to Japan’s Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture and Industrial Safety and Health Act as well as Europe’s REACH*2 We will continue to enhance our system for managing chemical substances in order to ensure even more meticulous management of these substances.
- *1 “Molded articles” here refers to all molded articles that have a defined shape with dimensions that can be measured. This applies to molded products and parts of devices, electronic components, paper, packaging materials, etc.
- *2 See the website glossary page.
●Chemical Substance Management System
Internal Quality Auditing and Daily Inspection/Review
For the quality audit for fiscal 2022, we continued to carry out the ‘Monozukuri’ Audits (see below), a multifaceted auditing system launched in 2017 by the Corporate Production Management and Engineering Division with the view to building a safe and reliable (personnel, facilities, environmental, and quality) approach to monozukuri.
The Corporate Quality Assurance Promotion Department inspects and examines operations daily from customers’ perspectives through support of R&D activities at research departments (participation in DR, cooperation with FMEA, etc.) and support of production activities at each business department (change control, FEMA, FTA, Why-Why Analysis, and Further Investigation, participation in quality meetings and DR, maintaining/managing quality information and quality data, and checking the appropriateness of complaint countermeasures, etc.). It also carries out activities to raise awareness about quality improvement.
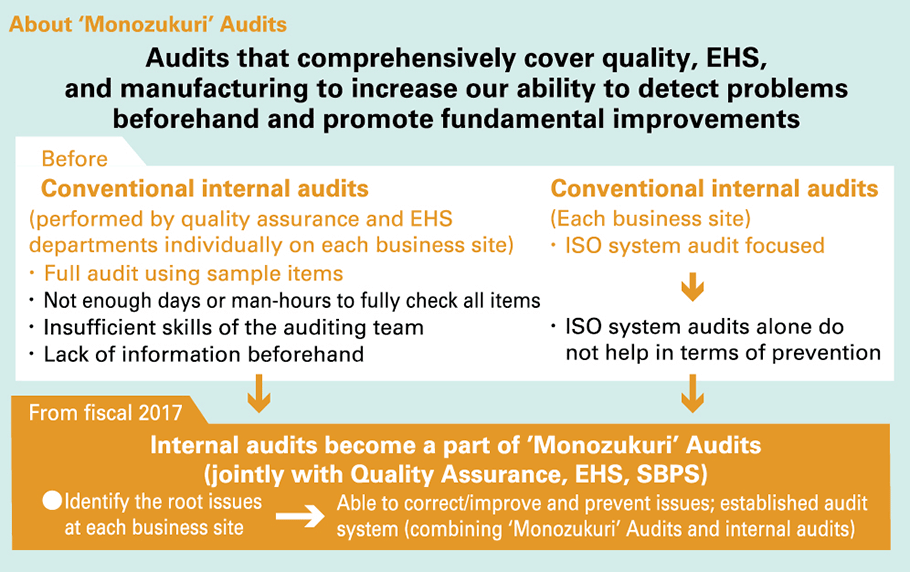
‘Monozukuri’ Audits
Purpose and method of the ‘Monozukuri’ Audits
Conventional audits that have been conducted for quality, environment, and safety for each business site made it difficult for us to identify the root issues each business site is faced with, due to factors such as insufficient man-hours spent on the audits, inadequate skills of the auditors, and a lack of information provided beforehand. Furthermore, the internal audits conducted by each business site focused on the management system, and it was not possible to successfully link the activities from the extraction of essential issues to the prevention of recurrence and prevention. We built an audit system (combining ‘Monozukuri’ Audits and internal audits) that is able to identify the root issues at each business site, correct and improve the situation, and prevent occurrence and recurrence of issues.
| (1) | Audits will be conducted in greater detail by updating check sheets to inspect and cover audit points that could pose or predict problems in terms of quality, EHS, or production that occurred last year. |
|---|---|
| (2) | Internal audits conducted by each business site provide education and instruction in advance to Internal Auditors for investigating the real cause and developing countermeasures, in order to make corrections/improvements with a PDCA cycle for issues carefully identified with audits. |
| (3) | ‘Monozukuri’ Audits will be run by the Corporate Production Management & Engineering Division to inspect internal audit results, the status of corrective actions or improvements, important matters, and involve follow-ups from a multifaceted approach, with a view to building a safe and reliable (personnel, facilities, environmental, and quality) approach to monozukuri. |
| (4) | Material issues identified in Monozukuri Audits will be rolled out laterally to other departments and business operators (inspections and corrective actions), with the resulting follow-up activities providing guidance for proper operation of monozukuri processes based on daily activities at each business site. ‘Monozukuri’ Audits will inspect internal audit results, the status of corrective actions or improvements, and important matters, and also involve follow-ups, etc. |
In fiscal 2022, we established and implemented an educational plan in order to strengthen education for Internal Auditors by dividing into finely differentiated steps the process starting before internal auditing and proceeding beyond ‘Monozukuri’ Audits.
Results of ‘Monozukuri’ Audits
In fiscal 2022, we conducted audites at four domestic business sites under direct control (the Shizuoka, Kanuma, Amagasaki and Utsunomiya Plants), three subsidiaries (Kyushu Sumitomo Bakelite Co., Ltd., Akita Sumitomo Bakelite Co., Ltd., and SB Kawasumi, Oita Business Site), overseas at SBS(Sumitomo Bakelite Singapore Pte.Ltd.) in Singapore.
We also conducted remote audits in response to the COVID-19 pandemic. While there were some issues with taking a flexible approach with remote audits, we were generally able to achieve our objectives through detailed prior discussions with the business sites. In fiscal 2023, we will continue to improve our systems to enhance internal audits.
Topics More
- 2024/02/01 Sustainability Sumitomo Bakelite Co., Ltd. announces its establishment of “Human Rights Policy for the Group of Sumitomo Bakelite Co., Ltd.”
- 2023/10/31 Sustainability Integrated Report 2023 of Sumitomo Bakelite Co., Ltd. has been issued.
- 2023/06/21 Sustainability Selected as an iSTOXX® MUTB Japan Platinum Carrier 150 Index
- 2022/10/31 Sustainability Integrated Report 2022 of Sumitomo Bakelite Co., Ltd. has been issued.
- 2022/10/14 Sustainability Sumitomo Bakelite Co., Ltd. Receives Gold Medal in EcoVadis Sustainability Assessment
- 2022/05/27 Sustainability Participation in the 30by30 Alliance for Biodiversity
 Inquiry
Inquiry