
Gut-liver co-culture system using BioStellar™ Plate:
a novel evaluation platform for simultaneous assessment of drug absorption and metabolism
Introduction
In drug development, animal testing has long been essential for evaluating drug efficacy and safety. However, ethical concerns and challenges in translating results to humans due to species differences are increasingly noted. In response, in vitro models are emerging as viable alternatives, with “Microphysiological Systems (MPS)”, gaining attention as next-generation platforms that closely mimic human physiological environments.
Sumitomo Bakelite Co., Ltd. has commercialized the BioStellar™ Plate, an on-chip pump-type multi-organ MPS developed by Professor Hiroshi Kimura at Tokai University.1 This MPS enables the connection of multiple cells and tissues, allowing for the reproduction of inter-organ interactions and the simulation of complex biological functions in vitro.
This application note presents a gut-liver co-culture system using the BioStellar™ Plate. By co-culturing intestinal and liver cells, the system enables more accurate reproduction of in vivo drug absorption and metabolism than single-culture methods. Further optimization is expected to improve replication of in vivo conditions and broaden its application as a powerful tool for advancing drug development.
Experimental overview
The BioStellar™ Plate is an MPS featuring a structure where two wells are connected by a flow channel. Perfusion culture can be easily performed by rotating a micro-stirrer bar within the flow channel. By incorporating cell culture inserts or coverslips, it is possible to co-culture up to four different cell types (Fig. 1 and 2).
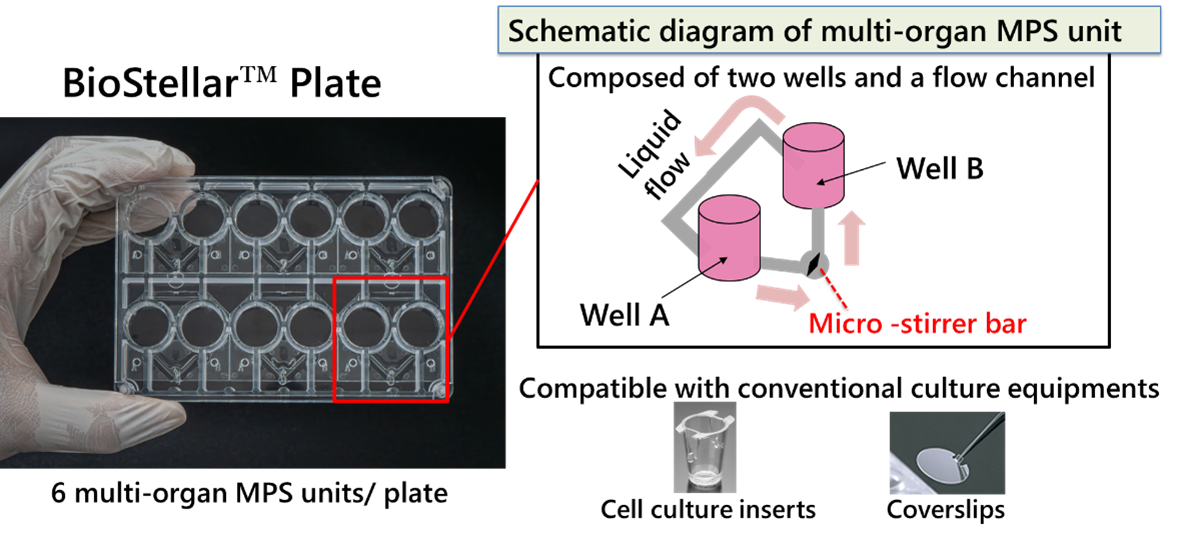
Fig. 1. Schematic Diagram of the BioStellar™ Plate
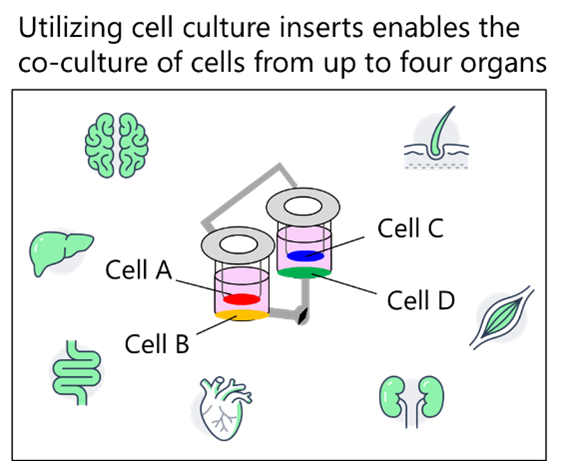
Fig. 2. Conceptual Diagram of the Co-culture System
The intestine and liver are vital organs for drug absorption and metabolism, making them crucial for predicting oral bioavailability. Previous research has demonstrated that co-culturing intestinal and hepatic cells on the BioStellar™ Plate enhances cellular functions, including improved hepatocyte metabolic activity.2 In this application note, we comprehensively evaluate drug absorption and metabolism within the gut-liver co-culture system and developed a platform that more accurately reflects in vivo drug behavior compared to single-culture systems (Fig.3).
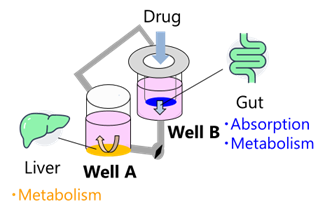
Fig. 3. Conceptual Diagram of the Gut-Liver Co-culture System
Experimental Procedure
(1) Pre-culture
Human iPS cell-derived intestinal epithelial cells (F-hiSIEC™, FUJIFILM Corp.) were used as gut cells and cultured on cell culture inserts (Transwell®, Corning) for 11 days. Liver cells derived from humanized liver chimeric mice (HepaSH®, BIOPREDIC International) were used as liver cells and cultured on coverslips (Cell Desk LF1, Sumitomo Bakelite) for 5 days (Fig. 4).
(2) Gut-liver co-culture
The pre-cultured F-hiSIEC™ (on cell culture inserts) and HepaSH® (on coverslips) were transferred to the BioStellar™ Plate, and a gut-liver co-culture was conducted for 2 days (Fig. 3 and 4). Perfusion culture was performed using a stirrer device set at a rotation speed of 2500 rpm. As controls, monocultures of gut and liver cells were also conducted.
(3) Drug evaluation
As a model compound, (S)-(+)-Clopidogrel Sulfate (hereinafter referred to as Clopidogrel) was used. This compound is known to be absorbed in the intestine and metabolized in the liver by the CES1 enzyme into an inactive form. In this experiment, the drug was added to the interior of the cell culture insert (hereinafter referred to “inside the insert”) for the gut-liver co-culture and gut-monoculture, and to wells A and B (hereinafter referred to as “inside the wells”) for liver-monoculture. Media samples were collected at 0, 1, 2, 4, 6, 24, and 48 hours after the start of co-culture. The concentrations of Clopidogrel and its metabolite, Clopidogrel carboxylic acid, were measured using LC-MS/MS (Fig. 4).
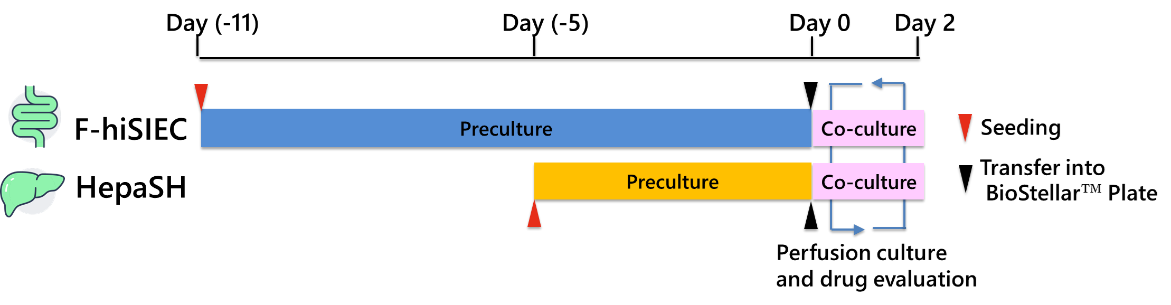
Fig. 4. Experimental Schedule
Results and discussion
Both the co-culture (Fig. 5A) and the gut-monoculture (Fig. 5B) exhibited a decrease in drug concentration inside the insert and an increase inside the wells. These results indicate that the co-culture, similar to the gut-monoculture, enables the evaluation of time-dependent permeability behavior mediated by gut cells.
Regarding liver metabolism, an increase in metabolite concentration inside the wells was observed in both the gut-liver co-culture (Fig. 5A) and the liver-monoculture (Fig. 5C). The metabolite-to-drug ratio was comparable to that in the liver-monoculture (Fig. 6), suggesting that liver cell function is maintained in the co-culture system. Moreover, in the gut-liver co-culture (Fig. 5A), the metabolite concentration increased more slowly compared to the liver-monoculture (Fig. 5C). This suggests that the gut-liver co-culture system allows for the time-dependent evaluation of drug absorption in the gut followed by liver metabolism, as the drug permeates from the insert to the well.
Furthermore, the nearly identical drug concentrations in wells A and B (Fig. 5A, 5B) confirm that the solution in the BioStellar™ Plate is uniformly mixed through perfusion.
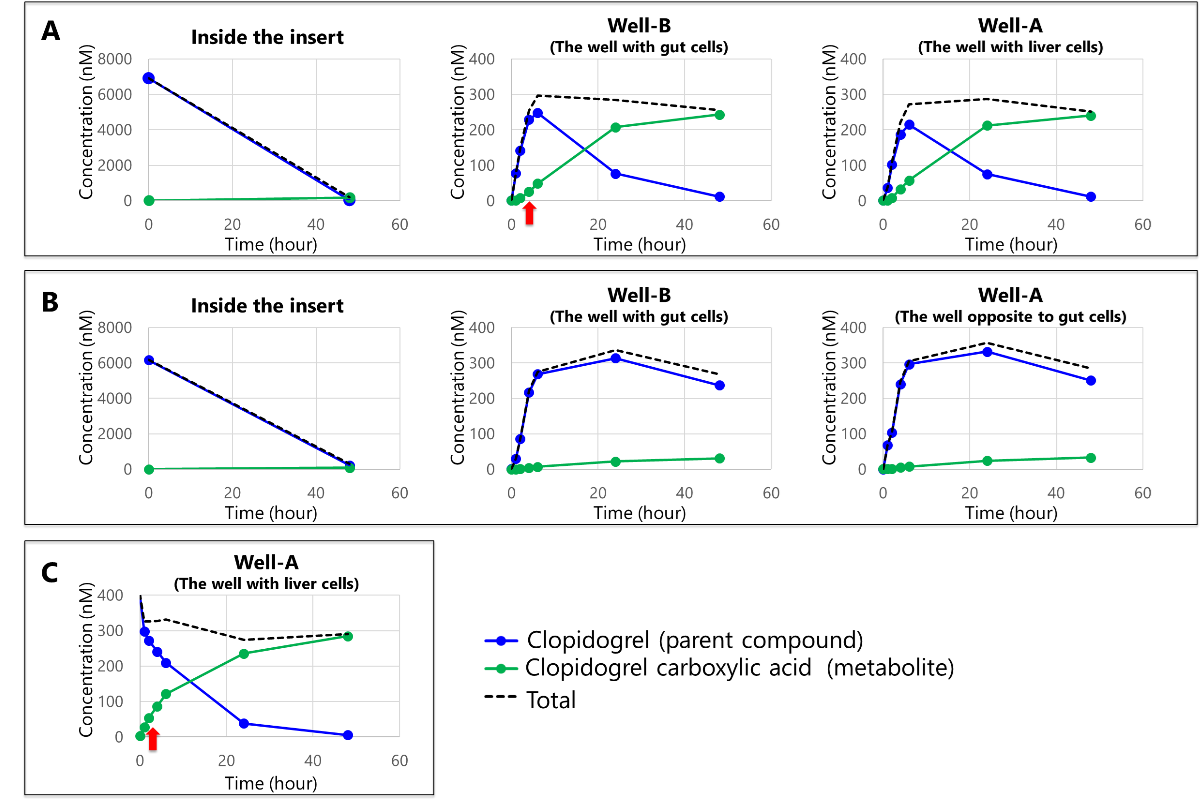
Fig. 5. Concentrations of Clopidogrel and its metabolites inside the insert and wells: (A) Gut-liver co-culture, (B) gut- monoculture, (C) liver-monoculture
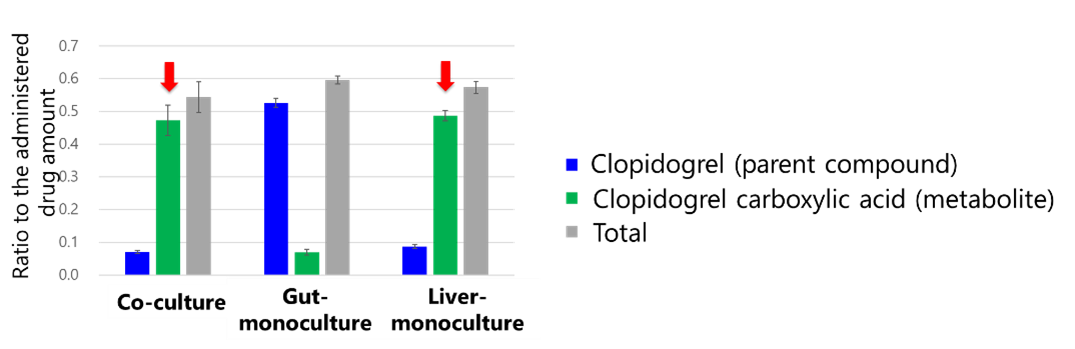
Fig. 6. Total amounts of Clopidogrel and its metabolites
Conclusion
In this study, we developed a gut-liver co-culture system using the BioStellar™ Plate to evaluate drug absorption and metabolism. The results obtained with the model compound demonstrated that the system reflects both drug absorption and metabolism, suggesting that this system enables more accurate evaluation of in vivo drug behavior compared to single-culture systems.
The gut-liver co-culture system using the BioStellar™ Plate enhance the in vivo reproducibility of drug absorption and metabolism and is expected to serve as an important tool for supporting drug discovery. Moving forward, we aim to evaluate drugs with various absorption and metabolic profiles and optimize the culture system by comparing the results with human clinical data.
Acknowledgements
The research was supported by the Japanese Agency for Medical Research and Development (AMED) (Grant No. JP17be0304201, JP22be1004201). And this study was conducted in collaboration with the Sakai Laboratory (The University of Tokyo), the Kato Laboratory (Kanazawa University), and the Kimura Laboratory (Tokai University). We sincerely thank everyone involved.
References
1) Shinha, K., Nihei, W., Nakamura, H. et al. (2021). Micromachines 12, 1-15
2) Kurniawan, D. A., Leo, S., Inamatsu, M. et al. (2024). PNAS Nexus 3, 1-10
Product information
BioStellar™ Plate
The BioStellar™ Plate is a multi-organ MPS designed for easy perfusion culture by rotating an integrated micro-stirrer bar with a dedicated device. By combining it with commercially available cell culture inserts, up to four types of cells can be co-cultured simultaneously. It features an open system structure with well sizes equivalent to those of a 24-well plate, ensuring operability comparable to standard culture equipment. Additionally, the plate does not use PDMS, resulting in minimal drug adsorption and reduced loss during evaluation.
Cell Desk LF
The CellDesk LF is a plastic coverslip designed to achieve low background fluorescence. It is surface-treated for adherent cells, making it suitable for cell fixation and specimen preparation. Its low autofluorescence properties make it ideal for immunochemical staining and observing intracellular localization using fluorescent markers.
| Product Number | Product Name | Contents | Price |
|---|---|---|---|
| BS-X9607 | BioStellar™ Plate | 2 plates / case | ASK |
| MS-92132 | Cell Desk LF1 | 240 (24×10) / case | ASK |
*For any inquiries, quotes, or orders regarding the products, please feel free to contact us directly.
E-mail: s-bio_inquiry@ml.sumibe.co.jp

